Consulting
Consulting
Working with clients to speed up submissions.
Aquila Solutions has over 10 years of experience working with biologic and pharmaceutical companies as a consultant and publisher for their submissions to the FDA.
The Consulting Services we offer include, but are not limited to:
Get In Touch
Lifecycle Planning and Consultation
Our consulting team can aid clients in planning and managing the lifecycle and submission schedule of their applications from the beginning pre-IND meeting requests to the post marketing requirements of their products.
Sequence Viewer Software
We have developed a proprietary eCTD viewer software, named “Altair,” which gives you a comprehensive overview of your entire submission, sequence by sequence, and has new features in development. For more information, please visit the Support page.
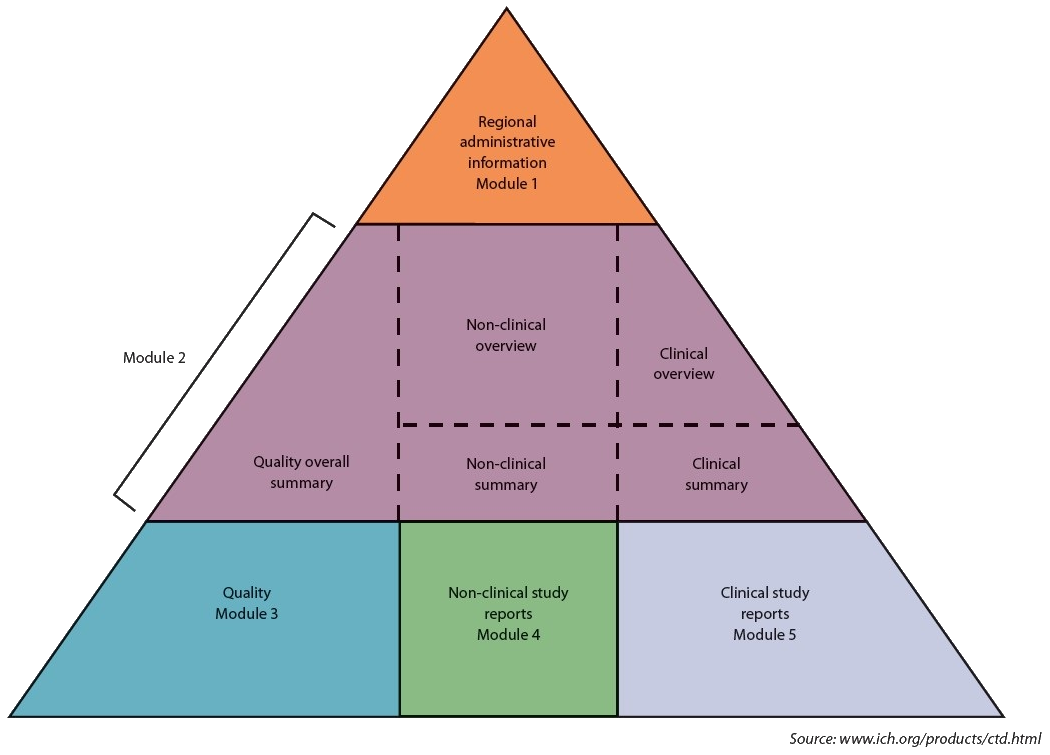
eCTD Structure from FDA
eCTD Templates
With our combined 40 years of experience in eCTD Publishing, we have developed a set of templates that can be used to help accelerate your submissions. These guidelines are an excellent starting point for a new submission or a new sequence in a submission! For more information, please visit the Support page.
Technical Assessments
Aquila allows clients to be confident in their new product purchases by offering a full lifecycle assessment of any existing applications. Also, Aquila offers technical assessments and validation of many sponsor software purchases currently on the market.

Sample eCTD Template
To request eCTD template information or check Aquila’s availability to guide your submission, please contact us.